Ocean Acidification – The Other CO2 Problem
Ocean acidification (OA) has been described as “global warming’s evil twin” and is considered by many scientists to be one of the greatest environmental challenges to marine organisms in the 21st century. Long-term records have shown a decrease in average ocean pH of 0.1 units since the beginning of the industrial age (from 8.21 to 8.10), and pH is expected to decrease a further 0.3 to 0.4 units by 2100, resulting in seawater that contains 150% more H+ than present (Box 1).
How do the oceans become more acidic?
It is estimated that between 30-40% of the CO2 released into the atmosphere as a result of burning of fossil fuels has been taken up by the oceans. In the equation below we see that increasing dissolved CO2 [CO2(aq)] results in the production of more hydrogen ions (H+):
CO2(atmos) ↔ CO2(aq) + H2O ↔ H2CO3 ↔ H+ + HCO3- ↔ 2H+ + CO32- (Equation 1)
With more H+, the pH of seawater decreases and becomes more acid, hence the term ocean acidification (Box 1).
Of importance for animals and plants that require carbonate (CO32-) to make skeletons, the greater availability of H+ leads to increased formation of bicarbonate (HCO3-), leaving lower concentrations of CO32- in a high-CO2 ocean. Organisms that require CO32- to form their calcium carbonate (CaCO3) skeletons include molluscs (e.g. mussels, oysters, paua, pteropods), crustaceans (e.g., lobsters), echinoderms, corals, bryozoans and some species of seaweeds. With less carbonate available in seawater to form skeletons, OA can lead to thinning of shells and/or reduced rates of growth.
What are the impacts of ocean acidification on marine ecosystems?
Considerable research has been undertaken in the past 15 years on the impacts of ocean acidification on marine ecosystems. It is becoming increasingly clear that the effects of OA are complex and that to understand the effects of OA we need to consider within the same experiments the effects of other climate change variables (e.g. seawater temperature, salinity) in what are called multi-stressor experiments. This is required as, for example, increased seawater temperature may counteract the negative effects of OA on the physiology and growth of many marine species.
Early reports on the effects of OA focused on two marine groups with calcium carbonate skeletons (CaCO3) – plankton species, such as coccolithophores, foraminifera and pteropods, as well as the reef-building corals. In experimental OA studies both of these groups are affected by the low availability of the carbonate they need to build their skeletons, and the impacts of more acid seawater dissolving the existing skeleton away.
Laboratory experiments have also shown that the early stages of development – embryos, and larvae – of a wide range of marine invertebrates are also susceptible to OA. In pH conditions lower than normal seawater, larval stages are usually smaller, have lower survival, and grow more slowly. To complete development these embryos and larvae may have to spend longer periods of time in these phases, leading to higher mortality and with the potential to be carried by currents to areas of unsuitable habitat. Lower survival of early developmental stages therefore has flow-on effects to adult populations – if less juveniles recruit to a local population, then over time we might expect to see a decline in population numbers. This is of particularly importance for species that are subject to recreational or commercial fisheries or used in aquaculture.
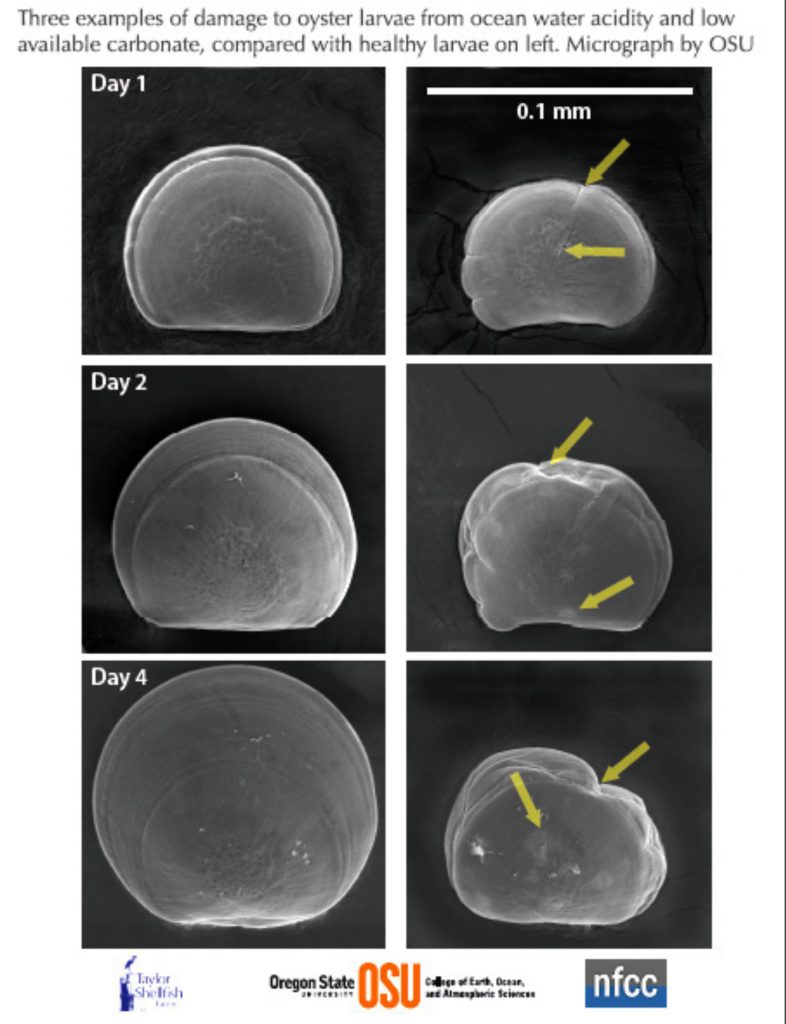
As an example, the scanning electron microscope images by Elizabeth Brunner and Dr. George Waldbusser from Oregon State University show Pacific oyster larvae are affected by acidic, unfavourable chemistry of ocean water (right) compared to healthy larvae of the same age raised in favourable water chemistry. The impacts of OA are seen with larvae having smaller shells, and changes to the form and structure of the shell. Similar results have been seen in OA studies in New Zealand of the commercially important Greenshell mussel, cockle, paua and flat oyster (see Capson and Guinotte 2014).
While much research has focused on marine organisms with shells, research also suggests that fish and other vertebrates with internal skeletons can be affected by lowered pH in surprising ways. For example, several species of tropical reef fish have impaired ability to discriminate between chemical cues, and evidence for neurological dysfunction when in OA conditions – this influences the ability of individuals to recognize the danger from a predator, but can also affect hearing, learning and the ability to school.
The ecosystem effects of Ocean Acidification.
Natural volcanic CO2 vent fields, where CO2 bubbles directly into the ocean, can provide information on the long-term effects of high-CO2 and the accompanying OA conditions on the entire ecosystem. Such CO2 vents are common in the Mediterranean, especially around Italy and Greece, and in Papua New Guinea. Data collected in Italy shows that as you move from normal pH (8.1–8.2) to low pH in the area of CO2 venting (mean 7.8–7.9) there is a shift from a typical rocky shore community with abundant calcareous organisms to a community lacking many calcareous species (e.g. hard corals and bryozoans), and with reduced abundances in other calcifying organisms such as sea urchins and coralline algae. Together with evidence of shell erosion in the areas of lower pH, these vents provide ecosystem-scale evidence for the negative impact of OA on marine calcifiers – and a view of the possible future. .
In the more tropical and coral-dominated vents in Papua New Guinea, areas of reduced pH (7.8) have reduced numbers of coral species, fewer coral recruits, lower abundance of the highly branched coral species, and dominance by massive Porites corals. No coral reefs have been found below a pH of 7.7. Loss of structurally complex corals results in potential loss of habitat for juvenile fish and many other marine invertebrates, and the low numbers of juvenile corals slows recovery of the coral reef after disturbances such as storms.
These venting sites suggest that a future high-CO2 ocean would have a very different community composition than we see in the present day. Loss of coral diversity and the habitat that they provide for other reef species is also of concern because of the ecosystem services (e.g. coastal protection from sea level rise and increased storm severity) and economic importance of coral reefs for fishing, recreation and tourism.
The economic effects of Ocean Acidification.
The Pacific Northwest of the USA, where shellfish aquaculture is an important source of both jobs and income, is providing a case-study for the broader impacts of OA on marine coastal communities (see Capson and Guinotte 2014). Local production of oysters, clams and mussels have been severely impacted by OA with failures of natural recruitment of many species, and the inability of companies to produce larvae in their hatcheries for commercial on-growing. In this particular location, the additional input of CO2-rich water that upwells from depth in the summer months and nutrient run-off from land-based agriculture have led to a “perfect storm” that warns other countries dependent on shellfish aquaculture of the future impacts of OA.
To ensure the long-term future of aquaculture in the Pacific Northwest the industry has lobbied state government to provide funding for local monitoring of seawater pH, development of models for forecasting when the lowest pH waters will be present, laboratory research on the targeted species and ways of treating hatchery water. In the immediate term, better monitoring has allowed hatcheries to store seawater in holding tanks that can be used during periods of upwelling of CO2-rich water and is investigating if selective breeding can provide more resilient populations for aquaculture in a future high-CO2 ocean.
How will New Zealand be affected by Ocean Acidification?
All New Zealand waters will be affected by OA, but as more CO2 dissolves in colder waters (thus leading to a greater change in pH – Equation 1), the pH changes will be most marked in the cooler waters of the South Island and Sub-Antarctic islands.
We have very little long-term data on pH in New Zealand waters. A notable exception, and one of the only long-term records in the Southern Hemisphere, has been collected by scientists at NIWA/University of Otago. Their 60 km transect running across the continental shelf from Dunedin has shown an average pH drop of 0.02 units since the year 2000 (Currie et al. 2011). The biological impacts of OA in these cooler southern waters is expected to be evident within the next few decades (as early as 2040).
As in the Pacific Northwest, areas where there is upwelling of cold, CO2-rich waters from depth onto the continental shelf are more vulnerable to OA. These include areas such as the Hauraki Gulf which are important areas for shellfish aquaculture, and also subject to eutrophication from adjacent intensive dairying. We might expect to see similar problems emerging in New Zealand shellfish aquaculture in such locations with respect to poor recruitment, and slower growth rate of calcifying marine species – for more information see the recent report by Capson and Guinotte (2014). There is currently a sparse body of knowledge on the effects of OA on commercially important New Zealand species, although recent and future funding is expected to address some of these gaps.
Where to for New Zealand? A recent workshop and summary report on how New Zealand and, in particular, the shellfish aquaculture industry should respond to OA (Capson and Guinotte 2014) made several important recommendations. The first was the need to develop a coastal ocean acidification network to provide information on spatial and temporal variation in carbonate parameters at key locations around New Zealand – an initiative currently under development. However, most importantly the report suggests that scientists, industry representatives and members of the public need to articulate their concerns about OA to government, particularly given the importance in New Zealand of the “blue economy”.
Websites
Numerous web sites provide information on Ocean Acidification – these are very good ones:
- Ocean Acidification website
- The Ocean Acidification Module (NOAA)
- Acid Test: The Global Challenge of Ocean Acidification – 22 mins. Originally aired on Discovery Planet Green.
- Rob Dunbar: The threat of ocean acidification
- Baby oysters, the canary of the oceans: Andrew Dickson at TEDxAmericasFinestCity
References
- Capson, T.L., Guinotte, J., Eds. (2014). Future proofing New Zealand’s shellfish aquaculture: monitoring and adaptation to ocean acidification. New Zealand Aquatic Environment and Biodiversity Report No. 136. 42 p. Available at https://www.mpi.govt.nz/news
- Currie, K.I., Reid, M.R., Hunter, K.A. (2011) Interannual variability of carbon dioxide drawdown by subantarctic surface water near New Zealand. Biogeochemistry 104: 23–34.
- Doney, S.C., L. Bopp, Long, M.C. (2014). Historical and future trends in ocean climate and biogeochemistry. Oceanography 27(1):108–119.
- Waldbusser, G.G., Salisbury J.E. (2014). Ocean acidification in the coastal zone from an organism’s perspective: Multiple system parameters, frequency domains, and habitats. Annu. Rev. Mar. Sci. 6: 221–247.
This paper was peer reviewed by:
Associate Professor Abigail Smith, Head of Department of Marine Science, University of Otago; and Dr. Victoria Metcalf, Lecturer in Genetics at Lincoln University, specialising in fish and shellfish.
While the reviewers have provided comment on drafts of this article, they do not necessarily endorse it in its final form. The author is solely responsible for any errors and judgements that may exist in the published article.
v1.1, 15th June 2016
