The Importance Of Methane
Concern about the emissions of Greenhouse Gas (GHG) that causes Climate Change focuses primarily on carbon dioxide (CO2), which is an unavoidable consequence of burning fossil fuels.
However, methane (CH4) is an increasingly important contributor to Climate Change, because:
- The Global Warming Potential (GWP) used in GHG accounting underestimates the importance of methane, particularly in the short term;
- CH4 from some industrial sources is more amenable to mitigation than CO2;
- Concern about methane emissions from melting permafrost in the Arctic is increasing because these emissions have the potential to dominate Climate Change effects.
Global Warming Potential of CH4
Reporting of greenhouse gas emissions as CH4 is now generally based on one tonne of CH4 having a GWP equal to 25 tonnes of CO2. A CH4 GWP of 21 was established in the 1998 second assessment report of the International Panel on Climate Change (IPCC AR2). It was based on the best understanding at that time of the science of the relative cumulative climate effects of CO2 and CH4 emissions over the subsequent 100 years. Subsequent IPCC reports have increased that number The choice of a 100-year time horizon was arbitrary.
However, CH4 molecules emitted to the atmosphere are destroyed by oxidation on much shorter timescale than 100 years. In contrast CO2 molecules are stable in the atmosphere and persist until they are eventually absorbed by the ocean. This comparative importance of methane and CO2 was reflected in the fifth IPCC report (IPCC AR5).

The science underpinning the IPCC reports has moved on over the last 15 years, the chemistry of both CH4 molecules and CO2 molecules in the atmosphere is complicated and is still subject to some uncertainty, but it is now understood by the IPCC scientists that, over a 20 year time frame, the impact of CH4 is four times more significant than the 1998 basis, which is still used for international reporting and emissions trading.
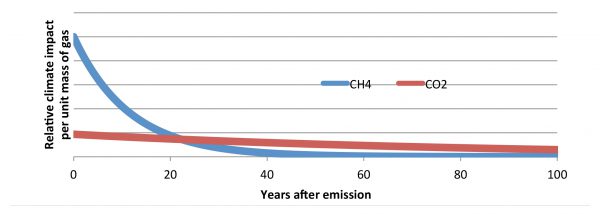
The following chart is derived from detailed analysis presented in the final draft of Chapter 8 of the fifth assessment report by Working Group 1 of the International Panel on Climate Change (IPCC- AR5, 2013). It shows that the climate impact of CH4 is much more significant over the first 20 years after discharge to air than over subsequent years. The IPCC AR5 report shows that the consequential integrated temperature rise effects peak about 10 year after the release of a pulse of CH4 into the atmosphere. It can therefore be argued that considering the climate impacts of CH4 over a 20 year time horizon is more meaningful than consideration over a 100 year time horizon.
Pollution control
CO2 emissions are an inevitable consequence of burning hydrocarbon fuels to release energy. In contrast, some CH4 emissions are an undesirable side effect of industrial activities and can sometimes be reduced by making the activity more efficient.
The most cost effective way of controlling CH4 emissions to air is to capture the emitted gas and use it as a fuel, whereby the CH4 is converted to the much less greenhouse-intensive CO2. If there is no local application for the use of CH4 as a fuel, then burning unusable CH4 in a flare is an effective way of reducing its greenhouse impact.
CH4 is potentially explosive within the range of 5% to 15% in air. To avoid hazard, CH4 capture systems for burning or flaring must keep the gas concentration well in excess of 15% in air. If that is not practical then dilution and dispersion of CH4 is used to ensure that the concentration of the gas in air is kept well below 5%, making combustion or flaring impractical.
Sources of methane
Ruminant animals
Ruminant animals eat vegetation that is decomposed in their first stomach where methanogenic bacteria produce CH4, which is breathed out by the animal. Choice of feed sources can reduce the CH4 production in farm animals and also increase the yield of meat/milk. About 80% to 90% of New Zealand’s CH4 emission inventory is attributed to cattle and sheep. Research into reduction of methane emission from ruminants has shown that CH4 production can be reduced with a strictly controlled diet in a feed lot. However, open paddock grazing as practiced in New Zealand is less amenable to strict diet control.
Rice paddies
Rice paddies and some other agricultural practices also generate CH4 from anaerobic fermentation underwater in wetlands. This CH4 source is also known as “marsh gas”.
Landfill gas
Landfill gas is generated when organic materials rot in the absence of air. Methanogenic bacteria convert the carbon in biodegradable materials to 50:50 CO2 and CH4. Some landfill gas can be captured and used or flared. A similar quantity of CH4 is generated by waste water treatment.
Biomass burning
Open burning of biomass is a significant source of CH4. The CH4/CO2 mole ratio is typically 0.5% to 1%. Hence deforestation and agricultural waste combustion have a secondary greenhouse gas impact. In contrast, the CH4/CO2 mole ratio from fossil fuel combustion is much lower.
Oil and Gas production
CH4 emissions arise from different stages of the oil and gas production, with or without fracking, and also from gas processing and delivery. The oil and gas industry claim that best practice can almost eliminate methane emissions from specific well completion activities. The US natural gas inventory reports average CH4 losses from well sites as less than 1% of gas produced. The GMI inventory (see below) for the global oil and gas industry, including processing and distribution losses, is equivalent to 2.7% of global natural gas production.
However, CH4 measurements in air over a gas fields in Colorado have indicated average well site emissions corresponding to 2% - 8% (Pétron et al. 2011). Atmospheric CH4 measurements in Utah, where environmental controls are less stringent, has indicated well- site emissions at 6% - 11% of production (Karion et al. 2013). Spatial and sectoral analysis of US-wide atmospheric methane data for 2007-8 (Miller et al 2013) determined aggregated methane emissions attributable to oil and gas production in Texas, Oklahoma and Kansas as 2% to 7% of gas production in those years. Gas distribution losses would be additional.
Pre-combustion emissions of CH4 would eliminate the greenhouse advantage of natural gas over coal for power generation if emissions are greater than about 5% of production, using the AR5 20-year GWP of 85.
Coal mining
CH4 is adsorbed onto coal underground, typically 0.5% of the energy content of the coal. Deeper coal is usually more gassy. When coal is mined the CH4 desorbs into the ventilation air in the mine causing a hazard, which has to be managed. Deep unmineable coal seams are sometimes drilled and hydraulically fractured (fracked) as a source of Coal Seam Methane, distributed as natural gas.

Global anthropogenic CH4 emissions (Mt CH4) - GMI
The Global Methane Initiative (GMI) has compiled a global inventory of anthropogenic sources of CH4 emissions (GMI – 2010). The total global CH4 emissions reported in the GMI inventory for 2010 was 322 million tonnes (Mt) per year, distributed between sources as shown in the above chart. Natural CH4 releases from wetlands, termites and geologic sources are estimated by GMI to be similar to anthropogenic CH4 emissions. So the total annual CH4 emission is over 600 Mt per year.
Using the AR5 20-year GWP of 85, 600 Mt per year would be equivalent to 51 Gigatonnes (Gt) of CO2 per year. The comparable global emission of CO2 from fossil fuels in 2010 was 34 Gt CO2 perr year (BP 2012) On this basis methane was a more significant greenhouse gas than CO2 in 2010.
The current atmospheric concentration of CH4 is about 1874 ppb in the Northern Hemisphere and 1758 ppb in the Southern hemisphere (IPCC 2013), compared with the pre- industrial level of 722 ppb. The CH4 content of the global atmosphere is about 5,000 Mt, of which about 550 Mt decomposes each year. Since 2006 the CH4 content of the atmosphere has been increasing by about 0.5% per year; similar to CO2.
Methane from the Arctic
The above CH4 sources are small compared with the potential release of frozen CH4 from beneath the Arctic Ocean. A recent paper in Nature (Nature 2013) reports “As the amount of Arctic sea ice declines at an unprecedented rate, the thawing of off-shore permafrost releases methane. A 50-gigatonne (GT) reservoir of methane stored in the form of hydrates, exists on the East Siberian Arctic Shelf. It is likely to be emitted as the seabed warms, either steadily over 50 years or suddenly.”
A group of concerned scientists has formed the Arctic Methane Warning Group (AMEG 2013) with a mission to warn the world of the potential for the Arctic region to cause global runaway climate change. In contrast a recent wide-ranging US National Academy of Sciences (NAS 2013) report on abrupt climate change considered Russian reports on arctic methane leaks, and concluded it was not an immediate significant hazard. However, those Russian reports are not up to date.
The very large additional source of sub-ocean CH4 has only recently been quantified. The cut off date for new information for IPCC-AR5 occurred before this phenomenon had been adequately observed and quantified. Therefore its effect on the global CH4 inventory and the climate change consequences of the release of large amounts of CH4 from the Arctic are excluded from the 2013 IPCC AR5 report and the GMI inventory. The instability of CH4 in the East Siberian Arctic Shelf is unusual. At present there is no other unstable large source of CH4 identified anywhere else.
If this Arctic CH4 release occurs steadily over 50 years at 1,000 Mt per year, as suggested in the Nature article, then the contribution by CH4 to Climate Change would be 2.5 times greater than estimated in the GMI data.
A worst case scenario
The IPCC AR5 report indicates that 4x1012 tonnes of additional CO2-eq in the atmosphere is likely to result in a 2oC higher global average temperature. A rapid 50,000 Mt release of CH4 in the Arctic, at the 20-year GWP of 85, would yield an additional 4x1012 tonnes of CO2-eq. That methane emission event alone would be sufficient to raise the global average temperature by 2oC.
However, the temperature rise could be localised to the Arctic region. The Arctic Circle comprises 4% of the surface of the planet. If the methane warming effect remains largely within the Arctic Circle then the localised temperature rise there could be some tens of degrees Celcius.
Greenland lies mostly within the Arctic Circle. After Antarctica, Greenland has the next largest mass of land-based ice in the world. The ice on Greenland is sufficient to raise global sea level by 7 metres, if it were all to slide into the sea.
A worst case scenario is that localised runaway warming occurs in the Arctic due to the feedback effect of rising temperatures releasing more frozen methane causing more regional temperature rise. Then the regional temperature rise causes the ice cap on Greenland to disintegrate and slide into the sea raising global sea level by up to seven metres. Could that worst case scenario play out in the Arctic Region within a decade or two? Nobody knows!
Suggested student assignment
Identify a source of methane emission (e.g. a gas leak) to the atmosphere that can potentially be avoided. Estimate the cost of a repair to avoid that methane emission. Calculate the climate change impact of that CH4 emission in terms of CO2 equivalents. Compare the cost of methane emission avoided per tonne of CO2-equivalent with the current carbon trading price.
References
- BP – 2012 – BP Statistical Review of World Energy – www.bp.com
- GMI – 2010 – www.globalmethane.org/documents/analysis_fs_en.pdf
- IPCC – 1998 – Second Assessment Report available – www.ipcc.ch
- IPCC – 2013 – Fifth Assessment Report available – www.ipcc.ch
- Karion et al. 2013 – Methane emissions estimate from airborne measurements over a western United States natural gas field. Geophysical Research letters. 40:16 pp4393-4397. 28 Aug 2013
- Miller et al. 2013 – Anthropogenic emissions of methane in the United States.
- NAS – 2013 – http://needtoknow.nas.edu/id/library/146/
- Nature 2013 – Issue 499; 25 Jul 2013; p401.
- Pétron et al. 2011 – Hydrocarbon Emissions Characterisation in the Colorado Front Range – A Pilot Study. NOAA. Journal of Geophysical research, Vol 117.
This paper was peer reviewed by
Dr Dave Lowe, Co-ordinator, New Zealand – Germany Science + Innovation Relationship; and Peter Barrett, Emeritus Professor of Geology, Antarctic Research Centre, Victoria University of Wellington.
While the reviewers have provided comment on drafts of this article, they do not necessarily endorse it in its final form. The author is solely responsible for any errors and judgements that may exist in the published article.
v5.1, 24 May 2016
